Welcome Biopharma Enthusiasts
Embarking on another thrilling week in biopharmaceutical innovation, let's dive deep into advancements shaping our industry's future.
What's in this issue:
-
🚀 Discover how circular RNA is revolutionizing therapeutics.
-
💊 Uncover Abivax's promising results in ulcerative colitis treatment.
-
💉 Learn about Sanofi's billion-dollar vaccine acquisition deal.
-
🤖 Explore advancements in AI-powered therapy with Slingshot AI.
Quote of the Day
"Innovation is the unrelenting drive to break the status quo and develop anew where few have dared to go." - Steven Jeffes
Latest Developments
🔬 Orbital Therapeutics Unveils First Monkey Data as It Enters In Vivo CAR-T Cell Therapy Race (2 minute read)
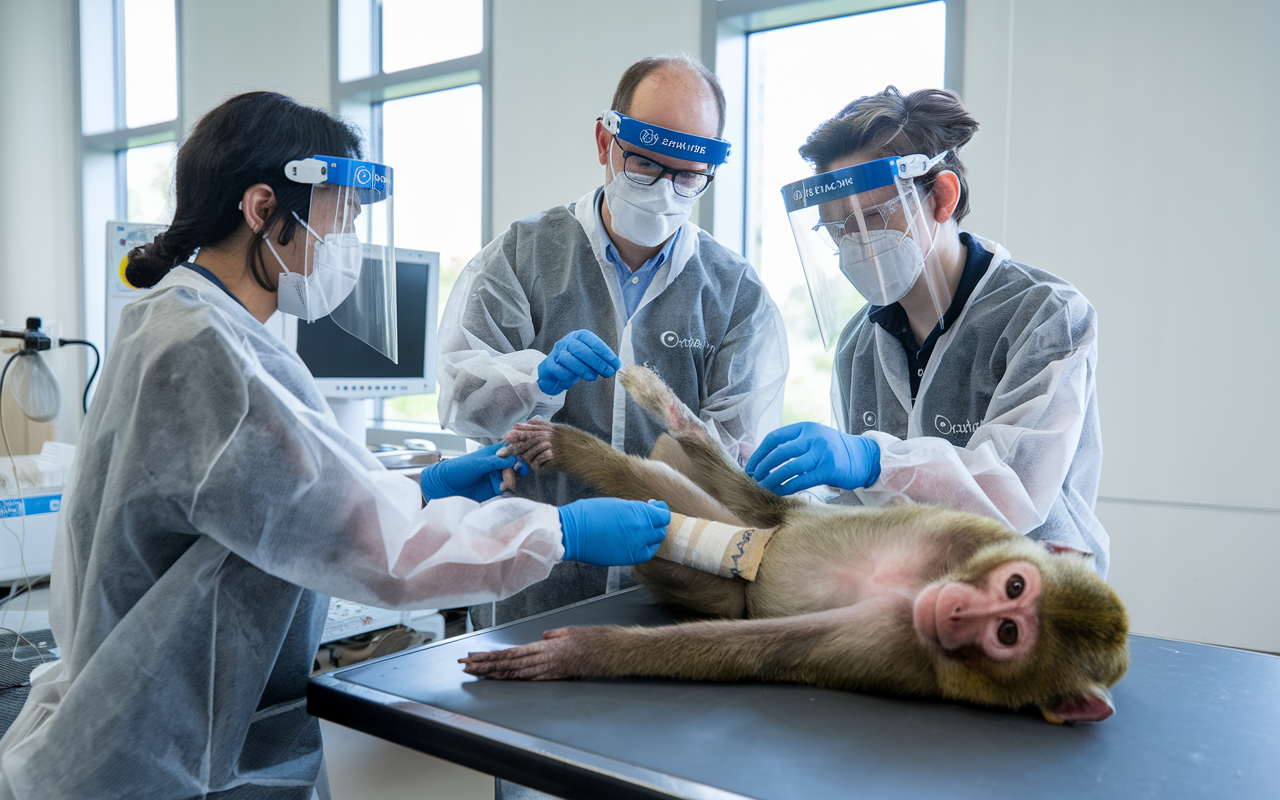
Rundown: Orbital Therapeutics, a pioneer in circular RNA technology, has shared its first preclinical data demonstrating enhanced durability of protein expression in mice compared to traditional mRNA. Their engineered circular RNA showed detectable protein levels at 17 days versus less than one day with linear mRNA.
Keypoints
-
🧬 Circular RNA shows improved stability over linear mRNA.
-
🚀 Potential to create "RNA-based programmable medicines" for various applications.
-
🤝 Partnerships with industry leaders and $270 million in funding secured.
Why it matters: This advancement could overcome mRNA's limitations, leading to more effective therapies with lower dosing requirements, and open new avenues in vaccines and treatments for autoimmune diseases.
🌟 Abivax Scores Major Win with Ulcerative Colitis Trials (2 minute read)
Rundown: Paris-based biotech Abivax announced that its experimental drug, obefazimod, led to significant symptom relief in patients with moderate to severe ulcerative colitis during Phase 3 trials, showing a 16.4% placebo-adjusted clinical remission rate.
Keypoints
-
💊 Obefazimod demonstrates compelling results in easing ulcerative colitis symptoms.
-
🧪 Successful Phase 3 trials could pave the way for new treatment options.
-
📈 Analysts hail the results as a "best-case scenario" for Abivax.
Why it matters: Ulcerative colitis impacts millions worldwide, and obefazimod's success offers hope for improved patient outcomes and quality of life.
💉 Sanofi to Acquire Vaccine Biotech in Billion-Dollar Deal (2 minute read)

Rundown: Sanofi has announced a $1.15 billion upfront deal to acquire Vicebio, gaining access to promising non-mRNA vaccine candidates targeting RSV, metapneumovirus, and parainfluenza.
Keypoints
-
🤝 Sanofi strengthens its vaccine pipeline with Vicebio acquisition.
-
🦠 Focus on critical respiratory viruses beyond COVID-19.
-
💡 Non-mRNA approach offers diversification in vaccine technologies.
Why it matters: With the spotlight on vaccine innovation, this acquisition positions Sanofi to address unmet needs in infectious diseases, potentially impacting global health significantly.
Question of the Day
❓ What biopharmaceutical innovation excites you the most?
Trending
🤖 Slingshot AI Launches Therapy Chatbot with $53M Funding
- Slingshot AI unveiled 'Ash', a therapy chatbot trained on sessions with human therapists, aiming to make mental health support more accessible.
🛡️ HHS and FDA Praise Move to Remove Artificial Colors from Food Supply
- A significant step towards healthier food options as major brands pledge to eliminate artificial, petroleum-based colors from their products.
Industry Insight
🧠 The Promise of Circular RNA in Therapeutics
Learn about circular RNA, a rising star in the RNA therapeutics landscape. Unlike traditional linear mRNA, circular RNA offers improved stability and durability, potentially leading to more effective treatments with fewer doses.
By understanding and harnessing circular RNA, biopharma innovators can develop next-generation therapies that may transform vaccine development, gene therapy, and treatments for various diseases.
Quick Hits
⚠️ FDA Rejects Replimune's Oncolytic Virus Therapy for Advanced Melanoma (2 minute read)
- The FDA issued a complete response letter to Replimune, citing concerns over trial design and enrollment for their viral-based skin cancer therapy.
📊 CROs Show Signs of Recovery but Warn of Uncertain R&D Funding (2 minute read)
- Contract research organizations report increased revenues despite earlier challenges but express caution about future research funding landscapes.
🩺 FDA Panel Elevates Concerns Over Antidepressant Use During Pregnancy (2 minute read)
- Experts argue that the risks associated with SSRIs during pregnancy may be greater than previously thought, prompting calls for updated guidance.
🚫 Roche Halts Some Shipments of Sarepta’s Duchenne Gene Therapy Outside U.S. (2 minute read)
- Following safety concerns, Roche pauses shipments of the gene therapy Elevidys in certain countries, impacting the Duchenne muscular dystrophy community.
🏥 Kentucky's Rural Cancer Care Model Threatened by Federal Cuts (2 minute read)
- Kentucky's successful initiative to improve rural cancer care faces challenges due to impending federal funding reductions.
Wrap Up
Thank you for joining us on this journey through the latest breakthroughs and developments in biopharma. Your curiosity and passion drive innovation forward. Stay informed, stay inspired, and let's continue shaping the future of healthcare together!
Warm regards,
Elliot Reeves | BioPharmaPulse
😊 How did you like today's email?
- 😍 Loved it
- 🙂 It was OK
- 😕 Could be better
