Welcome, BioPharma Enthusiasts!
Dive into the pulse of biopharma innovation! This issue uncovers groundbreaking developments that could redefine the future of medicine. Let's explore the transformative advancements shaping our industry.
What's in this issue:
- 🐷 United Therapeutics embarks on the first FDA-approved xenotransplantation trial.
- 🌬️ GH Research's inhaled treatment shows promise for depression.
- 💊 Pfizer's Braftovi confirms benefit in colorectal cancer.
- 🔬 Industry insights on precision medicine for complex diseases.
Quote of the Day
"The science of today is the technology of tomorrow." — Edward Teller
Latest Developments
🐷 FDA Approves First Xenotransplantation Clinical Trial as United Therapeutics Forges Ahead (2 minute read)
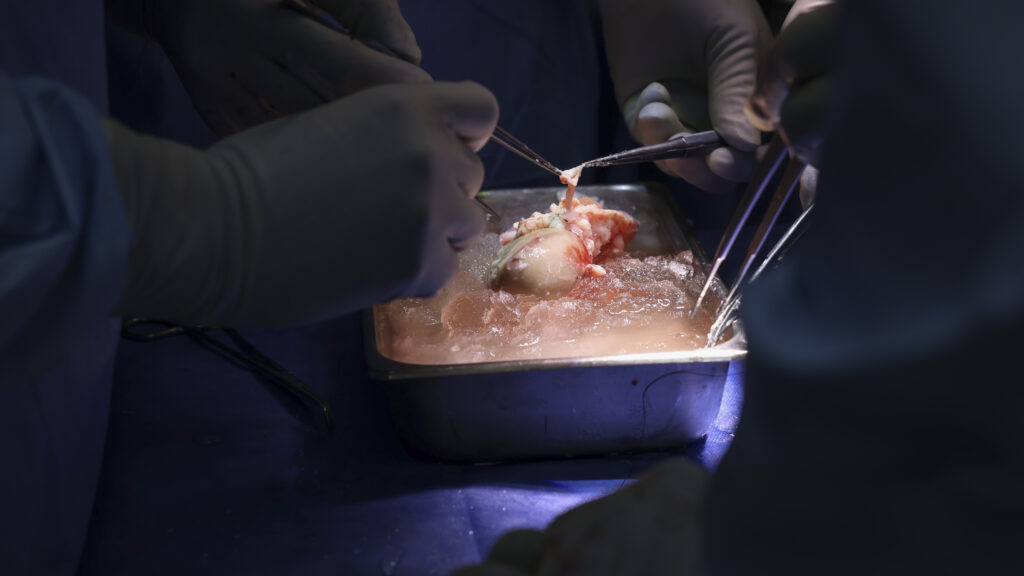
Rundown: United Therapeutics has received FDA clearance to begin the first clinical trial testing genetically modified pig kidneys in human patients. This landmark approval opens doors to potentially alleviate organ shortages faced by patients awaiting transplants. The trial will focus on patients with end-stage renal disease who have limited options.
Key Points
- 🚀 First FDA-approved clinical trial for xenotransplantation.
- 🧬 Utilizes gene-edited pigs to provide viable organs.
- 👥 Initial trial includes six patients with staggered dosing.
- 🔎 Aims to address the critical shortage of donor organs.
Why it Matters: This breakthrough could revolutionize organ transplantation, offering new hope to thousands on transplant waiting lists. Success in this trial may pave the way for broader applications of xenotransplantation in medicine.
🌬️ Irish Biotech's Inhaled Drug Reduces Depression in Phase 2 Trial (2 minute read)

Rundown: GH Research announced positive results from their Phase 2 clinical trial for an inhaled drug targeting treatment-resistant depression. The study showed significant reductions in depressive symptoms, offering a potential rapid-acting therapy for patients who have not responded to traditional treatments.
Key Points
- 💨 Inhaled therapy demonstrates quick onset of action.
- 🧠 Significant symptom reduction in resistant patients.
- 📊 58% remission rate by day eight of treatment.
- 🤝 Well-tolerated with mild to moderate side effects.
Why it Matters: This promising therapy could provide a much-needed option for those struggling with depression that hasn't responded to existing medications. Rapid relief can significantly improve patient outcomes and quality of life.
💊 Pfizer Drug Acquired in $11B Buyout Confirms Benefit in Colon Cancer (2 minute read)

Rundown: Pfizer's cancer drug Braftovi, acquired through the $11.4 billion acquisition of Array BioPharma, has confirmed benefits in treating patients with a specific type of colorectal cancer. The Phase 3 trial demonstrated that combining Braftovi with standard treatments delays disease progression and improves survival rates.
Key Points
- 🥼 Braftovi targets BRAF V600E mutations in colorectal cancer.
- 📈 Phase 3 trial shows delayed tumor progression.
- 💉 Combination therapy enhances standard treatment efficacy.
- 🔬 Reinforces value of targeted cancer therapies.
Why it Matters: These findings reinforce the significance of precision medicine in oncology, offering more personalized and effective treatment options. This success could lead to improved survival rates and set new standards in cancer care.
Question of the Day
🤔 What do you think is the most promising area of biopharmaceutical innovation?
- 🧬 Gene Editing and Genetic Therapies
- 🤖 AI-driven Drug Development
- 🦠 Immunotherapies and Vaccines
- 🌐 Other Emerging Technologies
Trending
🧬 Sionna Eyes Up $156M IPO to Fund Cystic Fibrosis Trials
- Sionna Therapeutics plans a $156 million IPO to advance its lead candidate into Phase 2 trials for cystic fibrosis, aiming to address unmet needs in this genetic disease.
🐦 Drugmakers Prep for Bird Flu Outbreak, Despite Continued Low Risk
- Pharmaceutical companies are preparing vaccines and treatments in response to avian flu outbreaks, enhancing pandemic readiness despite low current risk to humans.
Industry Insight
🔬 Delivering Precision Medicines for Complex Diseases
Half of the world's adult population is affected by metabolic dysfunction and related conditions like heart disease and diabetes. MultiOmic Health is leveraging AI and multi-omics data to develop precision medicines tailored to specific patient subpopulations.
By understanding the unique genetic and molecular profiles of patients, therapies can be more effective and personalized. This approach not only improves patient outcomes but also holds the potential to transform clinical trial success rates and healthcare economics.
Quick Hits
🖥️ DeepSeek Signals China’s Rising Influence in AI — and AI Drug Development (1 minute read)
- 🤖 DeepSeek, a Chinese AI firm, claims its technology rivals leading U.S. AI companies at a fraction of the cost, sparking discussions on global standards in AI-driven drug development.
💉 Scholar Rock Submits Biologics License Application for Apitegromab as a Treatment for SMA (1 minute read)
- 📄 Scholar Rock has submitted a BLA to the FDA for apitegromab, a promising treatment for spinal muscular atrophy, moving closer to providing a new therapeutic option for patients.
🧪 Affibody Regains Autoimmune Asset from Acelyrin (1 minute read)
- 🤝 Affibody recovers rights to izokibep after Acelyrin ends development, with plans to continue exploring its potential in autoimmune diseases.
💰 Sionna Seeks $135M IPO to Fuel Tests of Cystic Fibrosis Drug Candidates (1 minute read)
- 🚀 Complementing its IPO efforts, Sionna aims to accelerate clinical trials for innovative cystic fibrosis treatments.
Wrap Up
Thank you for joining us on this journey through the latest in biopharmaceutical innovation. Together, we're witnessing breakthroughs that could redefine healthcare and improve countless lives. Stay curious and keep exploring!
Warm regards,
Elliot Reeves | BioPharmaPulse
😊 How did you like today's email?
- 😍 Loved it
- 🙂 It was OK
- 😕 Could be better
