Welcome BioPharmaPulse readers
In the ever-evolving world of biopharmaceuticals, breakthroughs are happening at an unprecedented pace. This issue delves into the stories shaping the future of medicine and innovation. Let's explore the latest developments together.
What's in this issue:
- 🧬 Discover how gene therapy is restoring hearing in deaf children.
- 💊 Uncover Alnylam's bold steps toward tackling bigger diseases with siRNA.
- 🧠 Learn about a new treatment breakthrough for Tourette syndrome.
- 💡 See how biotech investors are adapting amid NIH funding cuts.
Quote of the Day
"The science of today is the technology of tomorrow." — Edward Teller
Latest News & Developments
🧬 Regeneron gene therapy helps deaf children hear in small study (4-minute read)
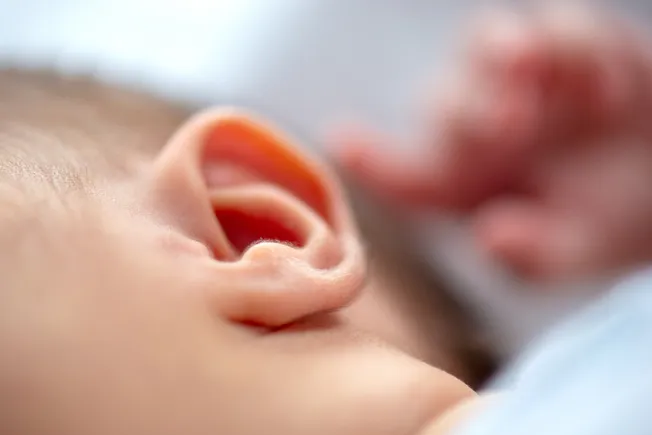
Rundown: Regeneron's experimental gene therapy has shown promising results in restoring hearing for children born profoundly deaf due to otoferlin gene mutations. In a small study, 10 out of 11 children experienced significant improvements, with some able to hear near-normal levels of sound, such as conversational speech.
Key Points:
- 👂 The therapy delivers a working copy of the OTOF gene to the inner ear.
- 🧬 Otoferlin is essential for converting sound vibrations into signals to the brain.
- 📈 Early results indicate potential for lasting improvements in hearing.
- 🚸 Children as young as 10 months showed notable benefits.
Why it matters: This breakthrough offers hope for a genetic form of deafness previously considered untreatable. Gene therapy could provide an alternative to cochlear implants and transform the lives of affected children by enabling natural hearing development.
💊 Alnylam eyes bigger diseases in more tissue types at ‘inflection point’ for siRNA (1-minute read)

Rundown: Alnylam Pharmaceuticals is expanding its focus, utilizing RNA interference (siRNA) technology to target more prevalent diseases beyond its current portfolio. The company believes it is at an inflection point where siRNA can address conditions across various tissues, opening new therapeutic possibilities.
Key Points:
- 🧬 siRNA technology silences specific genes responsible for disease.
- 🩺 Moving towards treating common diseases like hypertension and obesity.
- 🔬 Exploring delivery methods to target different tissues beyond the liver.
- 🌐 Aiming to broaden impact on global health challenges.
Why it matters: By advancing siRNA technology, Alnylam could revolutionize treatment options for widespread diseases. This approach has the potential to provide precision therapies with fewer side effects, marking a significant shift in how we address complex health issues.
🧠 Emalex dopamine blocker stops Tourette syndrome relapse in phase 3 win (1-minute read)

Rundown: Emalex Biosciences has announced successful Phase 3 trial results for ecopipam, a dopamine-1 receptor antagonist, in treating Tourette syndrome. The medication reduced relapse rates in both children and adults, paving the way for a new therapeutic option for this neurological disorder.
Key Points:
- 🧪 Ecopipam targets dopamine pathways differently from existing treatments.
- 🔄 Demonstrated significant reduction in tics and relapse rates.
- 👦👧 Effective in pediatric and adult populations.
- 📋 Plans to submit for FDA approval later this year.
Why it matters: Current treatments for Tourette syndrome often have limited efficacy or undesirable side effects. Emalex's novel approach offers a promising alternative that could improve quality of life for those affected by this condition.
Question of the Day
🤔 How optimistic are you about the potential of gene therapies in treating previously untreatable conditions?
Trending
💡 Where the money is: The top 100 venture investors in biotech
- Explore the key players driving investment in biotech, shedding light on trends that could shape the industry's future.
🌐 Indian drugmakers join forces to boost national biomanufacturing sector
- Discover how a consortium of Indian companies is aiming to strengthen the country's biomanufacturing capabilities.
Industry Insight
🔍 Navigating the Impact of NIH Funding Cuts on Biotech Innovation
Recent concerns have emerged regarding potential NIH research funding cuts and their implications for biotech innovation. Reduced grants could slow down early-stage research, affecting the pipeline of discoveries that fuel drug development.
By understanding the landscape, biotech companies and investors can explore alternative funding strategies, such as private partnerships or venture capital, to continue advancing scientific breakthroughs.
Quick Hits
📰 Kiniksa adds to Sjögren’s exodus with trial termination, axes AstraZeneca asset (1-minute read)
- Kiniksa Pharmaceuticals refocuses its efforts after discontinuing development of certain autoimmune assets to concentrate on cardiovascular therapies.
💼 Takeda fronts $46M for 2nd small molecule collab with BridGene (1-minute read)
- Takeda expands its partnership with BridGene Biosciences, enhancing its pipeline with innovative small molecule therapeutics.
💊 Eli Lilly makes Zepbound vials cheaper for self-paying patients (2-minute read)
- Eli Lilly introduces lower-cost options for its weight-loss drug Zepbound, making treatments more accessible to patients.
⚖️ Compounders sue FDA over declaring end of shortage for Novo's semaglutide (1-minute read)
- Compounding pharmacies challenge the FDA's decision regarding semaglutide shortages, highlighting ongoing debates in drug availability.
🧬 Lilly pays $10M upfront for Organovo’s FXR agonist program (1-minute read)
- Eli Lilly invests in Organovo's FXR agonist program, targeting conditions like ulcerative colitis and liver diseases.
Wrap Up
As we witness groundbreaking advancements in biopharmaceuticals, it's clear that innovation knows no bounds. Thank you for joining me on this journey through the heart of biotech progress. Together, we can stay informed and inspired by the remarkable strides being made every day.
If you found this edition insightful, feel free to share it with colleagues and friends who share our passion for biopharma innovation.
Warm regards,
Elliot Reeves BioPharmaPulse
😊 How did you like today's email?
- 😍 Loved it
- 🙂 It was OK
- 😕 Could be better
