Greetings, BioPharmaPulse Enthusiasts
Cancer continues to be one of the most formidable challenges in medicine, touching countless lives worldwide. Today, I'm thrilled to share groundbreaking developments that could reshape the way we approach cancer treatment.
What's in this issue:
- 🚀 Discover how a new gene therapy aims to revolutionize cancer immunotherapy
- 🌟 Learn about patients becoming insulin-free after innovative diabetes treatment
- 💊 Unpack GSK's bold move into autoimmune disease therapy
- 🧬 Explore the latest FDA approvals and what they mean for future treatments
Thought for the Day
"Every once in a while, a new technology, an old problem, and a big idea turn into an innovation." – Dean Kamen
Latest Developments in Biopharma
🚀 Paragon Spinout Joins Hunt for New Type of Cancer Immunotherapy (2-minute read)
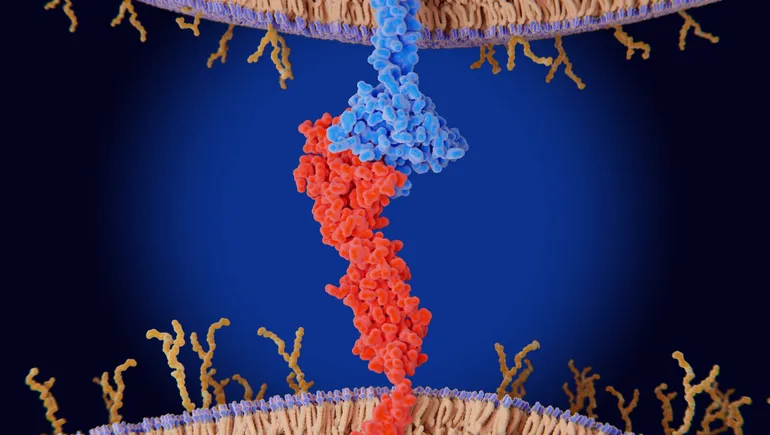
Rundown: Crescent Biopharma, a spinout from Paragon Therapeutics, has secured $200 million in financing to advance a new class of cancer immunotherapies. Their lead candidate uniquely targets both PD-1 and VEGF proteins, potentially offering a more effective treatment by simultaneously unleashing the immune system and inhibiting tumor blood vessel growth.
Key Points
- 🔬 Crescent Biopharma focuses on dual-pronged immunotherapies targeting PD-1 and VEGF.
- 💰 Secured $200 million from 17 major investment firms.
- 🧪 Early studies suggest improved efficacy over standard treatments like Merck's Keytruda.
- 🌐 Aims to be first to market with this novel combination approach.
Why it matters: This innovative therapy could redefine cancer treatment by addressing multiple mechanisms tumors use to evade the immune system. If successful, it may lead to more effective treatments with the potential to improve survival rates for patients worldwide.
🌟 Two Type 1 Diabetes Patients Are Insulin-Free After Eledon's Immunosuppressant and Islet Transplant (2-minute read)

Rundown: Eledon Pharmaceuticals announced that two patients with type 1 diabetes are now insulin-free following treatment with their experimental immunosuppressant combined with an islet cell transplant. This marks a significant step forward in the quest for a functional cure for diabetes.
Key Points
- 🩺 Patients achieved insulin independence after treatment.
- 💊 The immunosuppressant helps prevent transplant rejection without severe side effects.
- 🔬 Highlights the potential of islet cell transplants in treating diabetes.
- 🌟 Represents a promising alternative to lifelong insulin therapy.
Why it matters: This breakthrough offers hope for millions living with type 1 diabetes. By reducing the need for insulin injections, patients could experience improved quality of life and reduced long-term complications associated with the disease.
💊 GSK to Pay $300M to License Drug It Sees as Potential Lupus Treatment (2-minute read)

Rundown: GSK has licensed a bispecific antibody from Chimagen Biosciences for $300 million upfront, aiming to develop it as a treatment for autoimmune diseases like lupus. The drug targets faulty B cells by binding to CD19 and CD20 proteins, potentially offering a new therapeutic approach.
Key Points
- 🤝 GSK enters agreement with Chimagen Biosciences.
- 🧬 The bispecific antibody targets CD19 and CD20 on B cells.
- 🔬 Designed to deplete malfunctioning B cells involved in autoimmune diseases.
- 🚀 Plans to begin Phase 1 trials next year.
Why it matters: Autoimmune diseases like lupus have limited treatment options. This innovative approach could lead to more effective therapies with fewer side effects, improving outcomes for patients with chronic autoimmune conditions.
Question of the Day
🤔 How could dual-target immunotherapies change cancer treatment?
- 💡 They may enhance effectiveness by targeting multiple pathways
- 🔄 They might reduce side effects compared to single-target therapies
- 🌐 They could provide personalized treatment options
Trending Innovations
🧠 Modified Dosing Regimen of Eli Lilly's Alzheimer's Drug Leads to Significant Reduction in Brain Swelling
- Eli Lilly's Kisunla shows a 41% reduction in brain swelling with altered dosing, potentially improving safety for Alzheimer's patients.
🚀 Novartis Expands Scemblix Label to First-Line CML Patients
- FDA approves Scemblix for newly diagnosed chronic myeloid leukemia patients, offering a new first-line treatment option.
💉 Merck and Moderna Initiate Phase 3 Trial for Personalized Cancer Vaccine
- The trial evaluates the combination of mRNA vaccine V940 with Keytruda in non-small cell lung cancer.
Industry Insight
🧠 Understanding Dual-Target Immunotherapies
Dual-target immunotherapies represent a cutting-edge approach in cancer treatment by simultaneously targeting two different pathways that tumors use to survive and grow. For example, inhibiting PD-1 removes the "brakes" on the immune system, allowing it to attack cancer cells, while blocking VEGF disrupts the tumor's blood supply.
By combining these mechanisms, dual-target therapies aim to enhance treatment efficacy and overcome resistance that often develops with single-target treatments.
This strategy holds promise for more durable responses and could be a game-changer in oncology, leading to better outcomes for patients.
Quick Hits
🧪 EyePoint Sells $100M in Shares After Unveiling Eye Disease Data (1-minute read)
- EyePoint Pharmaceuticals raises funds following positive data for their drug-device candidate in diabetic macular edema.
🧬 PacBio Bets on 'Inflection Point' with $500 Long-Read Genome (1-minute read)
- Pacific Biosciences aims to make high-fidelity genetic sequencing more accessible to laboratories worldwide.
💉 Shionogi Touts Topline Data for COVID-19 Antiviral (1-minute read)
- Shionogi's antiviral ensitrelvir meets primary endpoint in late-stage trial for post-exposure COVID-19 treatment.
💡 Nektar Announces Positive Results for Rezpegaldesleukin in Skin Diseases (1-minute read)
- Nektar Therapeutics reports efficacy of rezpegaldesleukin in Phase 1b studies for inflammatory skin conditions.
🌐 Ionis Pharmaceuticals Granted FDA Fast Track for Alexander Disease Treatment (1-minute read)
- Zilganersen receives Fast Track designation, expediting development for this rare neurological disorder.
Wrap Up
Thank you for joining me on this journey through the latest innovations in biopharma. The advancements we're witnessing today not only fuel hope for better treatments but also inspire continuous pursuit of knowledge in this ever-evolving field.
Stay curious, stay informed, and let's continue to explore the breakthroughs that shape the future of healthcare.
Warm regards,
Elliot Reeves | BioPharmaPulse
📣 How did you like today's email?
- 😃 Loved it
- 🙂 It was OK
- 😕 Could be better
