Welcome BioPharmaPulse Readers
Welcome to another edition of BioPharmaPulse! In this issue, we delve into groundbreaking developments shaking up the biopharmaceutical landscape. From major industry partnerships to innovative therapies making strides in clinical trials, there's plenty to explore. Let's dive in and discover what's shaping the future of biopharma together.
What's in this issue:
- 💰 Novartis makes a billion-dollar move in the fight against Huntington's disease.
- ⚡ Innovative electric field therapy shows promise against pancreatic cancer.
- 🧬 FDA evaluates safety concerns over a gene therapy for a rare neurological disorder.
- 💡 Discover why radiopharmaceuticals are facing a talent shortage and what it means for the industry.
Quote of the Day
"Innovation is the calling card of the future." – Anna Eshoo
Latest Developments
💰 Novartis pays $1B upfront for PTC's Phase 2 Huntington's drug (2 minute read)

Rundown: Novartis is making a significant investment in the fight against Huntington's disease by agreeing to pay $1 billion upfront to PTC Therapeutics for the rights to PTC518, an experimental Phase 2 drug. This partnership aims to bolster Novartis's neuroscience pipeline and address a neurodegenerative disease with high unmet medical needs. PTC518 is being tested in a 250-person clinical trial, with results expected early next year.
Key Points
- 🧠 Novartis seeks to enhance its neuroscience offerings with PTC518.
- 🤝 The deal includes up to $1.9 billion in milestone payments.
- 🔬 PTC518 targets Huntington's disease, a genetic disorder causing nerve cell degeneration.
- 📊 PTC will use the funds to expand its drug development and commercial activities.
Why it matters: This substantial investment highlights the industry's commitment to tackling challenging neurodegenerative diseases like Huntington's. The partnership could accelerate the development of a much-needed therapy, potentially improving the lives of thousands affected by this condition.
⚡ Novocure says electric field therapy extends life for pancreatic cancer patients by two months (2 minute read)
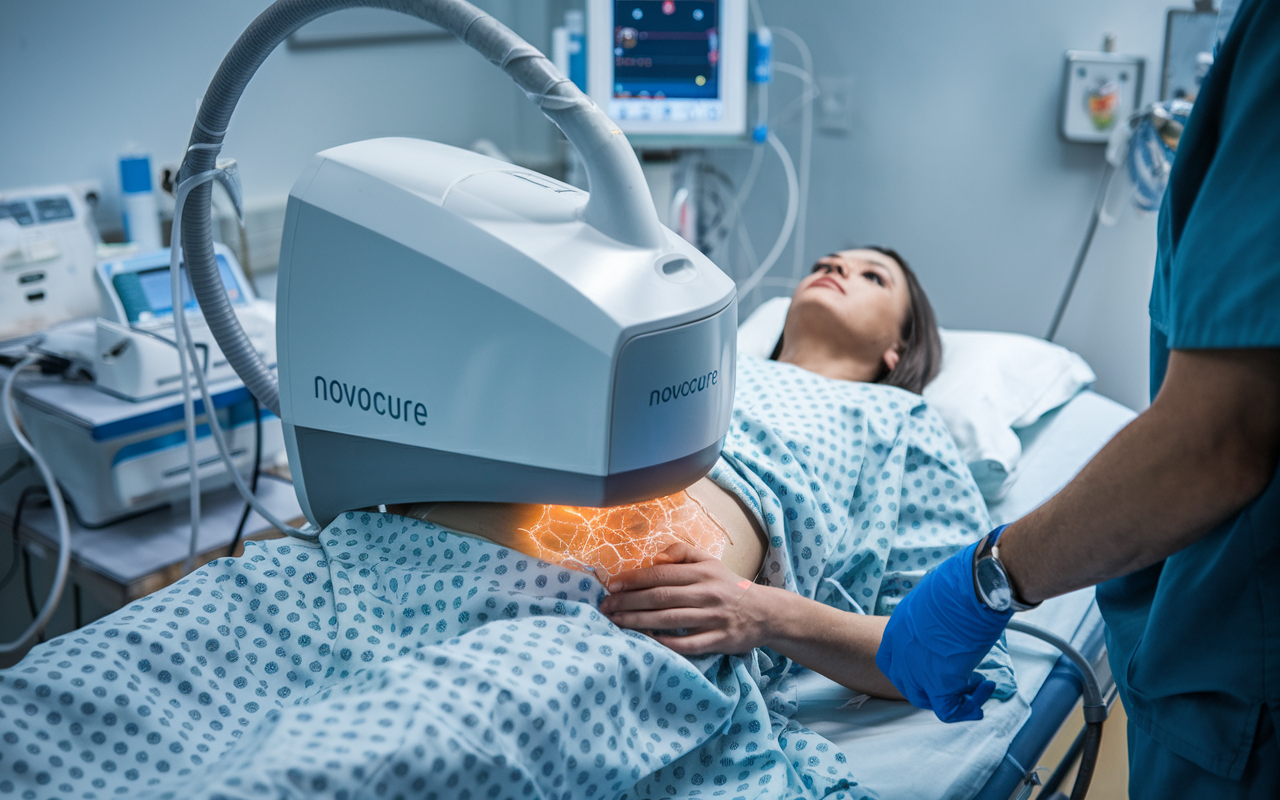
Rundown: Novocure's innovative electric field therapy, when combined with chemotherapy, has demonstrated a significant survival benefit for patients with locally advanced pancreatic cancer. In a Phase 3 trial, patients receiving the therapy lived an average of 16 months, extending life by two months compared to chemotherapy alone. Novocure plans to seek regulatory approval to make this therapy available to patients worldwide.
Key Points
- 🩻 Electric field therapy offers a non-invasive treatment option.
- 🎯 Targets cancer cells while sparing healthy tissue.
- 📈 Phase 3 trial shows statistically significant improvement in survival.
- 🌐 Novocure aims for global regulatory approvals.
Why it matters: Pancreatic cancer remains one of the most aggressive and lethal cancers. The addition of electric field therapy could represent a meaningful advancement in treatment, offering hope for improved survival rates in a patient population with limited options.
🧬 FDA considers 'regulatory action' for bluebird's Skysona after blood cancer reports (2 minute read)

Rundown: The FDA is evaluating potential regulatory actions following reports of blood cancer cases in patients treated with bluebird bio’s gene therapy, Skysona. Skysona is approved for cerebral adrenoleukodystrophy (CALD), a rare neurological disorder. Seven out of 67 patients developed blood cancer in clinical trials, prompting the FDA to recommend considering alternative treatments.
Key Points
- ⚠️ Seven cases of blood cancer reported post-Skysona treatment.
- 🧬 Skysona is a gene therapy targeting CALD.
- 📝 The FDA had previously issued a boxed warning for this risk.
- 🔎 Ongoing investigations into the therapy's safety profile.
Why it matters: Gene therapies like Skysona offer life-changing potential for patients with rare diseases. However, safety concerns underscore the need for rigorous evaluation and monitoring to ensure patient well-being. The FDA's scrutiny highlights the delicate balance between innovation and safety in biopharmaceutical advancements.
Question of the Day
🤔 What do you think is the most critical factor in advancing treatments for rare diseases?
- 🧬 Breakthrough scientific research
- 🤝 Collaborations between companies
- 💰 Increased funding and investment
Trending
🩺 Apogee targets Dupixent head-to-head next year as it outlines broad pipeline plans
- Apogee Therapeutics is planning a direct challenge to Dupixent with its own immunology and inflammation biologics pipeline, aiming to begin head-to-head trials next year.
💉 Biocon lands FDA approval for another Stelara biosimilar
- Biocon Biologics received FDA approval for Yesintek, a biosimilar to the immunosuppressive drug Stelara, expanding affordable treatment options for patients.
🌐 Sanofi charts biggest China investment to date with plans for €1B insulin 'manufacturing base' in Beijing
- Sanofi announces a €1 billion investment to build a new insulin manufacturing facility in Beijing, signaling commitment to expanding healthcare access in China.
Industry Insight
🔎 The Radiopharmaceuticals Talent Shortage
Radiopharmaceuticals are emerging as a promising frontier in cancer treatment, combining radioactive isotopes with targeting molecules to destroy cancer cells precisely. However, the industry faces a significant challenge: a shortage of specialized talent.
Radiopharmaceutical development and administration require experts skilled in handling radioactive materials, from researchers to healthcare professionals. This talent gap could hinder the progress and accessibility of these innovative therapies.
By fostering education and training programs focused on nuclear medicine and investing in talent development, the industry can overcome this hurdle, accelerating the advancement of radiopharmaceuticals and bringing cutting-edge treatments to patients in need.
Quick Hits
🧬 Merus drug shows 'unexpected improvement' in second-line head and neck cancer study (1 minute read)
- Merus reported positive results for its bispecific antibody drug in treating second-line head and neck cancer, showing potential as a new standard of care.
🧩 FDA rejects Applied filing for rare disease approval on clinical grounds, sinking stock (1 minute read)
- Applied Therapeutics faced a setback as the FDA rejected its application for a rare disease drug, citing insufficient clinical evidence.
💊 Diabetes advocacy group discourages use of compounded GLP-1 drugs (2 minutes read)
- The American Diabetes Association warns against using compounded versions of GLP-1 drugs like Wegovy and Zepbound due to safety and effectiveness concerns.
🏭 Agenus launches biologics manufacturing business to generate cash (1 minute read)
- Agenus announces the launch of a biologics CDMO to extend its cash runway, utilizing existing facilities to offer manufacturing services.
⚖️ Supreme Court tackles case involving FDA's oversight of flavored vapes (1 minute read)
- The Supreme Court heard arguments on whether the FDA overstepped its authority in denying marketing applications for flavored vaping products.
Wrap Up
Thank you for joining me on this exploration of the latest in biopharmaceutical innovation. It's an exciting time in our industry, with groundbreaking partnerships and promising therapies on the horizon. Your curiosity and engagement drive the advancements that make a real difference in patients' lives.
Stay tuned to BioPharmaPulse for your heartbeat on the cutting edge of biopharma. If you found this edition insightful, feel free to share it with colleagues and friends who are passionate about biopharmaceuticals.
Until next time, stay informed and inspired!
Warm regards,
Elliot Reeves | BioPharmaPulse
😊 How did you like today's email?
- 😀 Loved it
- 🙂 It was OK
- 😕 Could be better
