Welcome BioPharmaPulse Readers
*Dear BioPharmaPulse community,
Exciting breakthroughs are shaping the future of biopharmaceuticals, and I'm thrilled to share them with you.*
What's in this issue:
- 🔬 Sanofi and Teva's promising IBD therapy advances
- 👩⚕️ Pfizer welcomes former FDA deputy as Chief Medical Officer
- 🧬 Regeneron's gene therapy restores hearing in children
- 💡 Embracing humanity in epilepsy care
Quote of the Day
"The art of medicine consists in amusing the patient while nature cures the disease." - Voltaire
Latest Developments
🔬 Sanofi and Teva's IBD Drug Shows Promise in Phase 2 Trials (2 minute read)
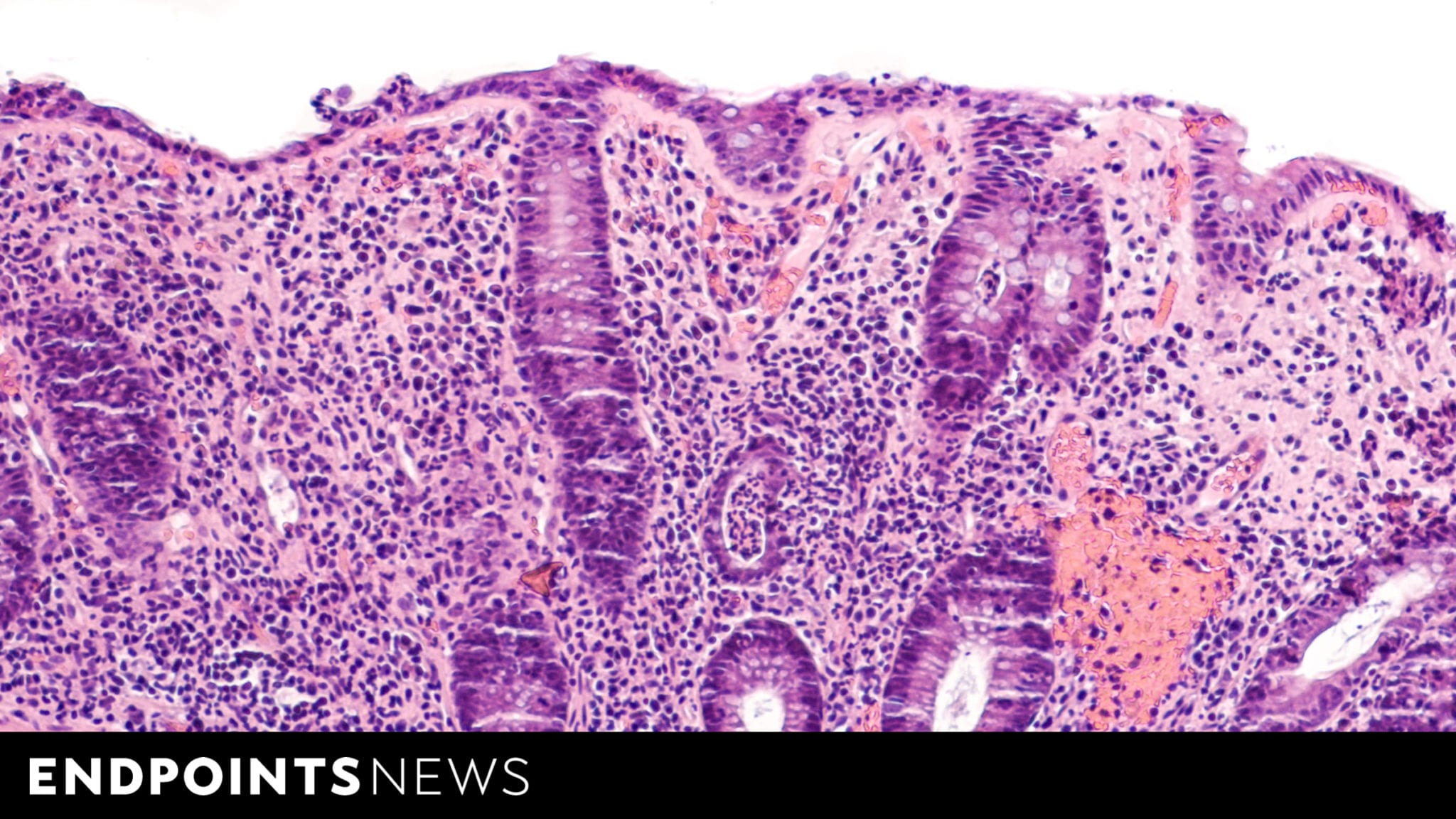
Rundown: Sanofi and Teva have revealed updated Phase 2b data for their investigational inflammatory bowel disease (IBD) therapy, showing significant promise. The drug could potentially reach blockbuster status with projected annual revenues of €1 billion by 2032.
Key Points
- 📈 Demonstrated strong efficacy in Phase 2b trials.
- 🤝 Collaboration strengthens development efforts.
- 💰 Potential blockbuster with significant market impact.
- 🏁 Positions the drug against competitors like Merck and Roche.
Why it matters: With millions affected by IBD worldwide, this advancement offers hope for improved therapies. It highlights the importance of strategic partnerships in accelerating biopharmaceutical innovation.
👩⚕️ Pfizer Appoints Former FDA Deputy Patrizia Cavazzoni as Chief Medical Officer (2 minute read)

Rundown: Patrizia Cavazzoni, the FDA’s former top drug regulator, is joining Pfizer as its new Chief Medical Officer. Cavazzoni previously led the FDA’s Center for Drug Evaluation and Research, bringing extensive regulatory expertise to Pfizer.
Key Points
- 🏥 Led drug approvals at the FDA.
- 💼 Will oversee Pfizer's regulatory and medical strategies.
- 🤝 Bridges regulatory and industry perspectives.
- 🌐 Highlights the movement between agencies and industry.
Why it matters: Cavazzoni's appointment could streamline Pfizer's regulatory processes and enhance innovation. Her insight is poised to benefit the development of new therapies, ultimately impacting patient care.
🧬 Regeneron's Gene Therapy Improves Hearing in Children with Rare Loss (2 minute read)

Rundown: Regeneron's investigational gene therapy has shown significant hearing improvements in 10 out of 11 children with a rare genetic condition causing hearing loss. This marks a promising step forward in treating sensory impairments with gene therapy.
Key Points
- 👂 Targets a specific genetic cause of hearing loss.
- 🧪 Early trials demonstrate notable efficacy.
- 🚸 Focus on pediatric patients lacking other treatments.
- 🔬 Showcases potential of gene therapy in rare diseases.
Why it matters: This breakthrough offers hope to families affected by genetic hearing loss and underscores the transformative potential of gene therapies in addressing unmet medical needs.
Question of the Day
🤔 How do you foresee gene therapy impacting the future of rare disease treatment?
Industry Insight
💡 Beyond the Diagnosis: Embracing the Humanity Behind Epilepsy Care
Understanding the lived experiences of those with epilepsy is crucial. In "Beyond the Diagnosis", we explore how empathy and curiosity drive innovation in patient care, emphasizing the importance of seeing patients as individuals with rich lives beyond their condition.
By embracing their stories, we can tailor interventions that truly resonate, improving not just symptoms but overall quality of life.
Quick Hits
🧬 23andMe's CEO Bids to Take Company Private for $74.7M (1 minute read)
- Anne Wojcicki, CEO of 23andMe, has submitted a higher offer to take the company private with New Mountain Capital. The move follows a previous bid that was rejected by the board, signaling ongoing strategic shifts within the company.
🌐 Amgen Plans Further $200M Investment in New Tech Facility in India (1 minute read)
- Amgen is set to invest an additional $200 million in its technology center in Hyderabad, aiming to expand its global footprint and leverage the region's growing biotech talent pool.
🔬 OS Therapies Seeks ADC Joint Ventures with US, China Peers (1 minute read)
- As OS Therapies prepares for FDA approval of its cancer asset, the biotech is exploring partnerships for its antibiotic drug conjugate technology, potentially spinning off this arm to focus on strategic collaborations.
Wrap Up
Thank you for joining me on this journey through the latest strides in biopharmaceutical innovation. It's inspiring to witness how our industry continues to push boundaries and improve lives. I invite you to share this newsletter with colleagues and friends who share our passion for advancing healthcare.
Until next time,
Elliot Reeves BioPharmaPulse
😊 How did you like today's email?
- 😍 Loved it
- 🙂 It was OK
- 😕 Could be better
