Hello BioPharma Enthusiasts!
Welcome to another edition of BioPharmaPulse! In this issue, we're delving into the latest developments and discussions shaping our industry. Let's explore the innovations and debates that continue to drive biopharmaceutical advances forward.
What's in this issue:
- 🧬 Unveiling the challenges faced by Sarepta Therapeutics
- 📝 Examining the controversies around FDA approvals
- 💭 Engaging insights from readers on critical health topics
- 🔎 Tips on navigating regulatory landscapes
Quote of the Day
"The art of medicine consists of amusing the patient while nature cures the disease." — Voltaire
Latest Developments
🧪 Sarepta’s Crisis Week; Prasad Overrides Reviewers Again; A New Study’s Clues About Alzheimer’s; and More (1 minute read)

Rundown: Sarepta Therapeutics faced significant hurdles this week, impacting its trajectory in the biopharma sector. Concurrently, discussions have arisen around Dr. Prasad's decision to override reviewer recommendations, highlighting debates in medical publishing. Additionally, new Alzheimer's research offers promising clues that could shape future treatments.
Key Points
- 🚨 Sarepta navigates critical challenges affecting its development programs.
- 🗣️ Debate sparked over the integrity of peer review processes.
- 🧠 Alzheimer's study provides new insights for potential therapies.
Why it Matters: These events underscore the dynamic nature of biopharmaceutical research and the importance of robust, transparent processes in advancing medical science.
📝 Opinion: A Sloppy Report on Mifepristone Is Being Used to Undermine the FDA — and the Biotech Industry (1 minute read)
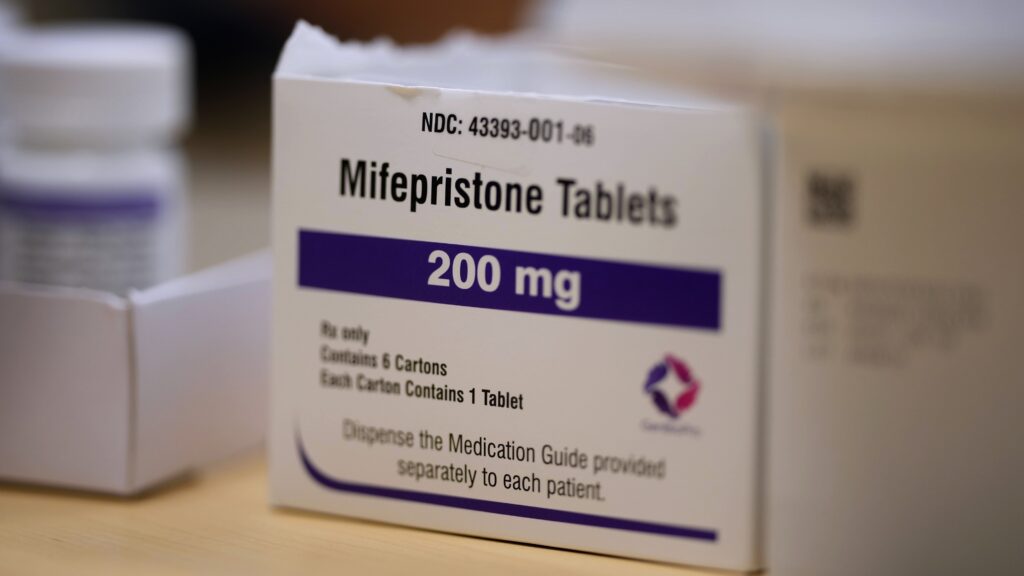
Rundown: A recent report challenges the FDA's long-standing approval of mifepristone, one of the most studied medications. Critiques highlight methodological flaws and political motivations behind the report, raising concerns about its impact on the biotech industry.
Key Points
- 📰 EPPC's report questions the safety of mifepristone despite extensive supportive data.
- 🏛️ Potential politicization of drug approval processes poses industry challenges.
- 📊 Over 25 years of data affirm mifepristone's safety and efficacy.
Why it Matters: Maintaining trust in regulatory agencies is crucial for innovation. Undermining the FDA's evidence-based processes can hinder the development and availability of vital therapies.
💬 Opinion: STAT Readers Weigh In on Marijuana-Related Vomiting, Drug Prices, and More (1 minute read)

Rundown: STAT's First Opinion platform highlights reader responses to pressing health issues, including cannabinoid hyperemesis syndrome, the complexities of drug pricing, and insights into spinal muscular atrophy symptoms.
Key Points
- 🗣️ Readers contribute valuable perspectives on emerging health concerns.
- 🌿 Discussions on identifying and managing cannabis-related conditions.
- 💰 Ongoing debates surrounding the factors influencing drug costs.
Why it Matters: Engaging with diverse viewpoints enhances our understanding of health challenges and promotes collaborative approaches to innovation and patient care.
Question of the Day
🤔 What's the most significant factor that can accelerate biopharmaceutical innovation?
- 🚀 Advancements in technology
- 🤝 Collaborations between industry and academia
- 🔍 Streamlined regulatory processes
Industry Insight
🧭 Navigating Regulatory Landscapes in Biopharma
Understanding the intricacies of regulatory requirements is essential for successfully bringing new therapies to market. Here's how:
- 📚 Stay Informed: Keep abreast of the latest guidelines from regulatory bodies like the FDA.
- 🤝 Engage Early: Initiate dialogue with regulators during the early stages of development.
- 🔄 Adaptability: Be prepared to adjust strategies in response to regulatory feedback.
By proactively addressing regulatory considerations, companies can mitigate delays and accelerate the delivery of innovative treatments to patients.
Quick Hits
🌐 Engaging Discussions on Global Health Issues (1 minute read)
- Readers share insights on diagnosing emerging syndromes and the worldwide impact of drug pricing.
🧠 New Clues in Alzheimer's Research (1 minute read)
- Recent studies shed light on potential pathways for Alzheimer's treatments.
🏛️ The Importance of Evidence-Based Approvals (1 minute read)
- Discussions around maintaining integrity in the drug approval process amid political pressures.
Wrap Up
Thank you for being part of our journey through the latest in biopharmaceutical innovation. Your curiosity and passion drive the advancements that make a real difference in healthcare. Feel free to share this newsletter with peers who share your commitment to progress.
Until next time,
Elliot Reeves | BioPharmaPulse
😊 How did you like today's email?
- 😍 Loved it
- 🙂 It was OK
- 😕 Could be better
